Solutions
Diagnostic uncertainty is constant, and the fear of missing something critical in the ED (Emergency Department) is ever-present.
Bacterial and viral infections are clinically indistinguishable, leading to over 40% antibiotic overuse and 20% underuse1,2.




LIAISON® MeMed BV® and LIAISON® BRAHMS PCT® (Procalcitonin) provide a timely and actionable diagnostic pathway from bacterial vs viral determination, to disposition, sepsis severity monitoring, and discharge.
1 MeMed survey of ED pediatricians (n=42).
2D. Wang, et al. Primary Care Respiratory Medicine (2021).

LIAISON® MeMed BV® helps confirm infection source and antibiotic need early, supporting clarity of patient disposition.
LIAISON® MeMed BV® is a novel immune-based protein signature test that differentiates the etiology of acute infections – viral or bacterial and can identify a co-infection.
It measures and computationally integrates the levels of three host proteins (TRAIL, IP-10, and CRP) to deliver a fast, easy-to-interpret score, indicating the likelihood of a bacterial or viral infection in 35 minutes.
How it works
Technology Description
We’ve advanced from one marker to three, utilizing a proprietary algorithm to deliver a more accurate and actionable score. Rather than identifying a specific pathogen, the test evaluates the body’s immune response to distinguish between bacterial and viral infections by measuring host markers in the blood.
Researchers analyzed thousands of serum proteins from patient samples to identify a signature of three key markers: TNF-related Apoptosis-Induced Ligand (TRAIL), Interferon Gamma-Induced Protein 10 (IP-10), and C-reactive protein (CRP). Each biomarker exhibits a distinct response pattern to viral versus bacterial infections, including differences in peak levels and timing.
A proprietary algorithm integrates these three markers into a robust score, effectively distinguishing bacterial from viral infections within 7 days of symptom onset.

Three Biomarkers + Machine Learning = Better Patient Care
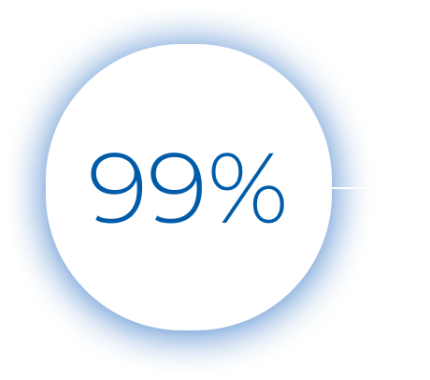
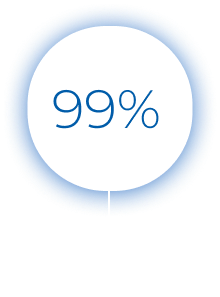
The test results are divided into five bins, each indicating an increasing likelihood of bacterial infection. Patients with a score of 0 to 35 have a viral infection over 99%* of the time. The viral bins accurately predict viral illness, making the test an effective rule-out tool for bacterial infections.
To be used on
Designed for both specialty and routine tests, LIAISON® XL and LIAISON® XS immunoassay analyzers help your laboratory handle multiple patients and tests simultaneously. LIAISON® systems are trustworthy, intuitive and deliver automated continuous operation with minimal user intervention. The result is reduced turnaround time, optimal cost management.

LIAISON® BRAHMS PCT® helps manage infection and sepsis progression, guiding antibiotic therapy.
Procalcitonin (PCT) is a biomarker that aids in diagnosing and managing bacterial infections and sepsis.
PCT may guide and monitor antibiotic treatment in various settings, including primary care, ED, and ICU.
Together with the trusted quality of B·R·A·H·M·S and the robust reliability of the LIAISON® analyzer, your laboratory can focus on what matters most: patient care.
Only B·R·A·H·M·S PCT assays offer unparalleled, evidence-based clinical performance to provide you the right guidance for every patient every time.
1.7M
ADULTS DEVELOP SEPSIS
IN THE U.S.
350K
DIE BECAUSE OF SEPSIS
Sepsis is the leading cause of death in U.S. hospitals, accounting for 35% of all in-hospital deaths.
Intended use
How it works
Kinetics of B·R·A·H·M·S PCTTM
In the presence of a bacterial infection, PCT levels will increase in the first 3 to 6 hours after the onset of infection. The levels will rise rapidly, peaking at 12 to 24 hours. The half-life of PCT is approximately 24 hours.1,2,3
Tracking PCT kinetics
PCT has prognostic implications, with higher levels correlating to the severity of the infection and rapidly declining levels after treatment, indicating a positive prognosis.4
Assessing PCT trends over time provides another key insight: patients whose PCT levels fail to decline under treatment may face therapeutic failure and increased mortality risk.4
In particular, it was demonstrated that PCT levels that decline less than 80% from the baseline within four days are associated with increased all-cause 28-day mortality—especially when the baseline PCT measurement is > 2.0 ng/mL.4
Unique kinetics of PCT are strong indicators of patients’ response to antibiotic therapy.1,2,3
1 Harbarth et al., American Journal of Respiratory and Critical Care Medicine, 2001.
2 Meisner M, Procalcitonin – Biochemistry and Clinical Diagnosis, 2010.
3 Müller et al., Critical Care Medicine, 2000.
4 Schuetz et al., Crit Care Med, 2017:781-789

For more information, please visit Thermo Fisher
Clinical Significance of Procalcitonin: Evidence Matters
PCT Values Rise in relation to sepsis severity and is the best indicator for severity of bacterial infection.
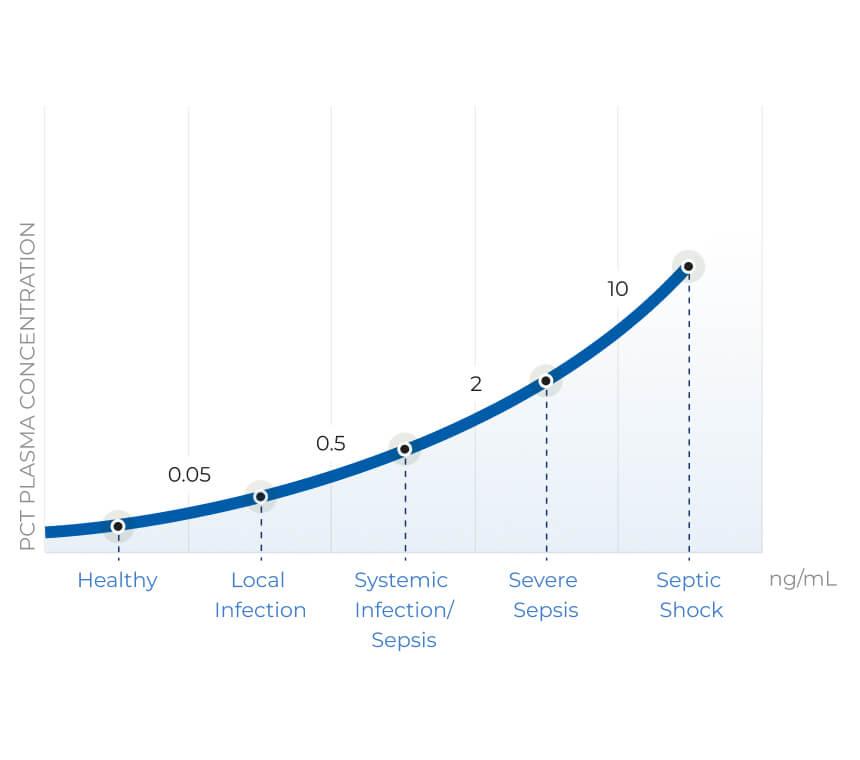
The combination of superior sensitivity, specificity, and fast kinetics sets PCT apart from other biomarkers when assessing bacterial infection and its severity. But not all PCT tests are the same; it’s essential to know how to identify a PCT assay that sustains improvement of patient care through proven clinical, economic, and analytical performance.
Only B·R·A·H·M·S PCT assays offer a substantial amount of clinical evidence and alignment to a gold standard1, ensuring continuous clinical performance.2, 3
PCT has been extensively documented in over 10,000 publications4, including reviews, clinical trials, meta-analyses, experts’ consensus5, and international and national guidelines. Almost all this evidence has been generated using Thermo ScientificTM B·R·A·H·M·S PCTTM assays.
This strong evidence from reproducible, randomized clinical trials supports the effectiveness and safety of PCT-aided antibiotic stewardship protocols, for which only B·R·A·H·M·S PCT assays have an approved indication.
1 Chambliss et al., AACC Guidance Document on the Clinical Use of Procalcitonin . J Appl Lab Med 2023; 8(3): 598-634.
2 Meisner M, Procalcitonin – Biochemistry and Clinical Diagnosis, ISBN 978-3-8374-1241-3, UNI-MED, Bremen 2010.
3 Harbarth S et al: Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. AM J Respir Crit Care Med 2001;164(3):396-402.
4 Müller B et al: Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Critical Care Medicine 2000;28(4):977-983.
5 Morgenthaler NG et al: Detection of procalcitonin (PCT) in healthy controls and patients with local infection by a sensitive ILMA. Clin Lab 2002;48(5-6):263- 270.
To be used on
Designed for both specialty and routine tests, LIAISON® XL and LIAISON® XS immunoassay analyzers help your laboratory handle multiple patients and tests simultaneously. LIAISON® systems are trustworthy, intuitive and deliver automated continuous operation with minimal user intervention. The result is reduced turnaround time, optimal cost management.

LIAISON® XL
Equip your laboratory with rapid, high-throughput testing using the Diasorin LIAISON® XL. This fully automated immunoassay analyzer delivers critical results—like cardiac markers, infectious disease panels, and thyroid profiles—with speed and accuracy. The LIAISON® XL helps emergency physicians make faster, more informed decisions, improving patient outcomes and ED efficiency.
Why choose LIAISON® XL
- Continuous loading
- Broad test menu
- Proven reliability
- Save space, reduce cost

LIAISON® XS
The LIAISON® XS brings fast, accurate testing to hospital labs—delivering critical results in minutes. With a compact design, broad test menu, and walkaway automation, it supports Emergency Department physicians with rapid, reliable data to guide urgent clinical decisions and improve patient care.
Why choose LIAISON® XS
- Rapid results, turnaround
- Efficient workflow management
- Enhanced diagnostic accuracy
- Compact and flexible design